

We have written many articles about atomic mass for Universe Today.
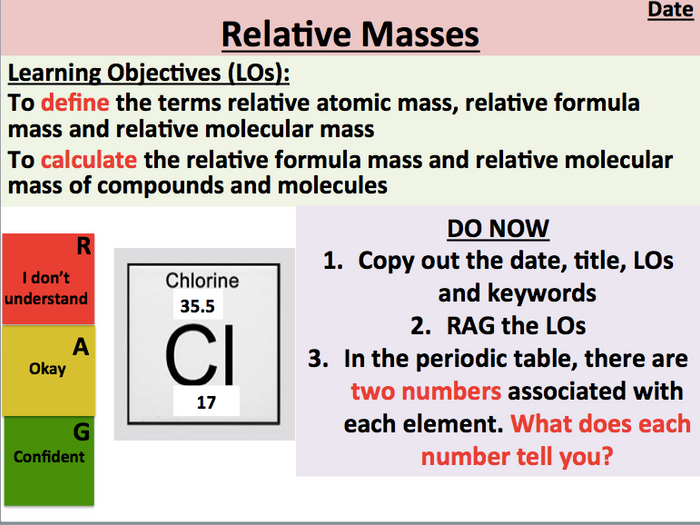
Both atomic and molecular masses are expressed by the AMU. One atomic mass unit is equal to 1.66 x 10-24 grams. The unit of measure for mass is the atomic mass unit (amu). Units of measure have been defined for mass and energy on the atomic scale to make measurements more convenient to express. With advances in our knowledge, even these terms may become obsolete in the future. The atomic mass of an element determines its atomic mass unit. The size and mass of atoms are so small that standard measuring units, while possible, are often inconvenient. Large variations can occur in elements with many common isotopes. Atomic weight is different from atomic mass in that it refers to the most abundant isotope in an element and atomic mass directly addresses a single atom or isotope.Ītomic mass and standard atomic weight can be so close, in elements with a single dominant isotope, that there is little difference when considering bulk calculations. It has been the source of much debate largely surrounding the adoption of the unified atomic mass unit and the realization that ‘weight’ can be an inappropriate term. This shift in wording dates back to the 1960’s. Atomic weight is being phased out slowly and being replaced by relative atomic mass. This is what is included in standard periodic tables. Material Properties Atomic Mass Isotopic Mass The size and mass of atoms are so small that the use of normal measuring units, while possible, is often inconvenient. Standard atomic weight is the average relative atomic mass of an element in the crust of Earth and its atmosphere. The atomic mass, or isotopic mass, is the mass of an atom.


 0 kommentar(er)
0 kommentar(er)
